Essay
First law of thermodynamics: The pV diagram shown is for 7.50 moles of an ideal diatomic gas taken through a cycle from a to b to c. The ideal gas constant is R = 8.314 J/mol ∙ K. 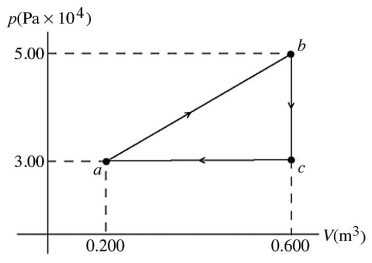 (a) What is the highest temperature reached by the gas during the cycle?
(a) What is the highest temperature reached by the gas during the cycle?
(b) What net work does the gas do during the cycle?
(c) How much heat is exchanged with the gas during part bc of the cycle? Does it enter or leave the gas?
(d) What is the change in the internal (thermal) energy of the gas during part bc of the cycle?
(e) What is the change in the internal (thermal) energy of the gas during the entire cycle?
Correct Answer:

Verified
(a) 208°C
(b) 4.00 ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
(b) 4.00 ...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: Molar heat capacities: A monatomic ideal gas
Q2: First law of thermodynamics: An ideal gas
Q3: First law of thermodynamics: During an adiabatic
Q4: First law of thermodynamics: In a thermodynamic
Q5: First law of thermodynamics: In an isochoric
Q7: First law of thermodynamics: An ideal gas
Q8: Types of thermodynamic processes: The process shown
Q9: First law of thermodynamics: A system has
Q10: First law of thermodynamics: When a fixed
Q11: First law of thermodynamics: When a fixed