Essay
An analytical chemist wanted to use electrolysis to determine the number of moles of cupric ions in a given volume of solution. The solution was partitioned into n = 30 portions of 0.2 milliliter each. Each of the n = 30 portions was tested. The average number of moles of cupric ions for the n = 30 portions was found to be 0.185 mole; the standard deviation was 0.015 mole. Calculate the following intervals: 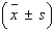 = ______________
= ______________ 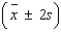 = ______________
= ______________ 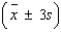 = ______________
= ______________
Describe the distribution of the measurements for the n = 30 portions of the solution using Tchebysheff's Theorem.
________________________________________________________
Describe the distribution of the measurements for the n = 30 portions of the solution using the Empirical Rule.
________________________________________________________
Suppose the chemist had used only n = 5 portions of the solution for the experiment and obtained the readings 0.18, 0.21, 0.20, 0.22, and 0.18. Would the Empirical Rule be suitable for describing the n = 5 measurements?
______________
Explain.
________________________________________________________
Correct Answer:

Verified
(0.170, 0.200); (0.155, 0.215); (0.140, ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q176: If two data sets have the same
Q177: A new manufacturing plant has 20 job
Q178: A distribution of measurements is relatively mound-shaped
Q179: Which measure of center is meaningful when
Q180: The following data represents the number of
Q181: Thirty-three students were asked to rate themselves
Q183: The value of the standard deviation may
Q184: The Empirical Rule and Tchebysheff's Theorem can
Q185: The standard deviation is a measure of
Q186: Measures of center are values around which