Multiple Choice
The acidity of a solution is determined by the concentration H of hydrogen ions. The formula is . The accompanying exponential formula is H = 0.1pH. Lower pH values indicate a more acidic solution. Normal rain has a pH of 5.6. Suppose acid rain has a pH of 4.3. How many times as acidic as normal rain is this? 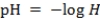
A) 2
B) 5
C) 10
D) 20
Correct Answer:

Verified
Correct Answer:
Verified
Q3: Suppose you are at the base of
Q4: What is the number of distinct real
Q5: The speed of sound at 0 degrees
Q6: A rectangular playground has the dimensions 20
Q7: The _ of a radioactive substance is
Q9: Which of the following is a quadratic
Q10: You have a 400-foot roll of fencing
Q11: For the linear function y = mx
Q12: The loudness of sound decreases as the
Q13: Radium-226 is subject to radioactive decay, and