Multiple Choice
Consider a sample of water in a closed, rigid container. Which of the following will indicate that the given physical process has reached equilibrium? 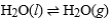
A) The mass of the container remains constant.
B) The vapor pressure in the container remains constant.
C) The volume of water in the container increases from its original level.
D) All of these indicate that the reaction has reached equilibrium.
Correct Answer:

Verified
Correct Answer:
Verified
Q9: In an energy diagram for an endothermic
Q10: In an equilibrium constant expression, what does
Q11: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8310/.jpg" alt="For the
Q12: Consider the following energy diagram for a
Q13: Which of the following is true of
Q15: A particular reaction has an equilibrium constant
Q16: If a chemist wishes to react a
Q17: If a chemist wishes to react a
Q18: Which of the following will change the
Q19: Why will increasing the temperature of a