Multiple Choice
Consider a sample of water in a closed container. When the physical process 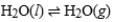 has reached equilibrium, what can we say about any specific water molecule?
has reached equilibrium, what can we say about any specific water molecule?
A) If it was present in the liquid phase, then it will always remain in the liquid phase.
B) If it was present in the vapor phase, then it will always remain in the vapor phase.
C) It will sometimes be in the liquid phase and sometimes be in the vapor phase.
D) If it was present in the liquid phase, then it will always remain in the liquid phase, and if it was present in the vapor phase, then it will always remain in the vapor phase.
Correct Answer:

Verified
Correct Answer:
Verified
Q81: What is the effect of increasing the
Q82: Consider the following energy diagram for a
Q83: For which of the following reactions is
Q84: In an energy diagram for an exothermic
Q85: A catalyst speeds up a chemical reaction
Q87: For the exothermic reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8310/.jpg" alt="For
Q88: Which of the following is true of
Q89: To which of the following systems does
Q90: Which of the following best describes the
Q91: In a particular chemical reaction, three bonds