Multiple Choice
What is the effect of adding CH3COOC2H5(g) to a container in which the following reaction has reached equilibrium? 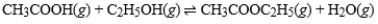
A) The reaction will shift from the left to the right.
B) The reaction will shift from the right to the left.
C) There will be no effect.
D) Whether the reaction will shift to the left or the right depends on the initial temperature.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Which of the following can be the
Q3: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8310/.jpg" alt="For the
Q4: For the reaction 2C → A +
Q5: Which of the following will change the
Q6: How does the rate of reaction change
Q8: Which of the following does not result
Q9: In an energy diagram for an endothermic
Q10: In an equilibrium constant expression, what does
Q11: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8310/.jpg" alt="For the
Q12: Consider the following energy diagram for a