Multiple Choice
Consider the following energy diagram for a reaction. 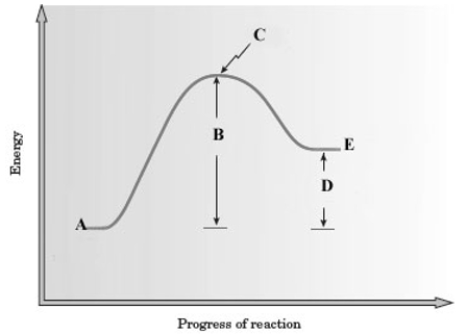 Which of the following is a correct interpretation of the graph?
Which of the following is a correct interpretation of the graph?
A) The energy of the products is less than that of the reactants, so the reaction is endothermic.
B) The energy of the products is greater than that of the reactants, so the reaction is endothermic.
C) The energy of the products is less than that of the reactants, so the reaction is exothermic.
D) The energy of the products is greater than that of the reactants, so the reaction is exothermic.
Correct Answer:

Verified
Correct Answer:
Verified
Q15: A particular reaction has an equilibrium constant
Q16: If a chemist wishes to react a
Q17: If a chemist wishes to react a
Q18: Which of the following will change the
Q19: Why will increasing the temperature of a
Q21: Which of the following is not true
Q22: A particular chemical reaction carried out at
Q23: Which of the following are generally included
Q24: When considering the effect of a catalyst
Q25: Solid sodium chloride and solid silver nitrate