Multiple Choice
Consider the following representation of a voltaic cell. 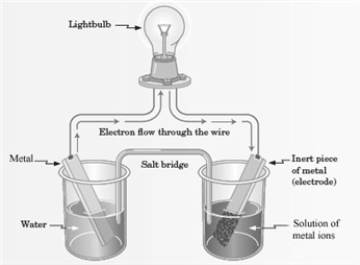 This voltaic cell is represented by the following reaction: Mg(s) + Cu2+(aq) → Cu(s) + Mg2+(aq)
This voltaic cell is represented by the following reaction: Mg(s) + Cu2+(aq) → Cu(s) + Mg2+(aq)
What is the identity of the "Metal" shown in the diagram?
A) Mg
B) Cu
C) Mg2+
D) Cu2+
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q120: Which of the following compounds has the
Q121: The atomic weight of copper is greater
Q122: 0.117 mol of a particular substance weighs
Q123: The atomic weight of copper is less
Q124: Which of the following sulfides is soluble
Q126: You have a sample of 1.204 ×
Q127: How many hydrogen atoms are there in
Q128: The temperature of an unknown substance weighing
Q129: Consider the following representation of a voltaic
Q130: The atomic weight of platinum is less