Multiple Choice
Consider the following representation of a voltaic cell. 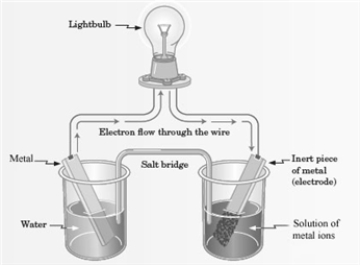 This voltaic cell is represented by the following reaction: Mg(s) + Cu2+(aq) → Cu(s) + Mg2+(aq)
This voltaic cell is represented by the following reaction: Mg(s) + Cu2+(aq) → Cu(s) + Mg2+(aq)
What is the identity of the ion in the solution shown in the diagram?
A) Mg
B) Cu
C) Mg2+
D) Cu2+
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: Which of the following definitions of reduction
Q12: You have a sample of 3.01 ×
Q13: Ethanol is produced industrially by the acid-catalyzed
Q14: Which of the following is the SI
Q15: What is the molecular weight of sucrose,
Q17: A black precipitate of copper sulfide, CuS,
Q18: The metabolism of one mole of glucose,
Q19: Which of the following is true of
Q20: When solutions of AgNO<sub>3</sub> and NaCl react,
Q21: In the industrial synthesis of acetic acid,