Multiple Choice
Consider the following representation of a voltaic cell. 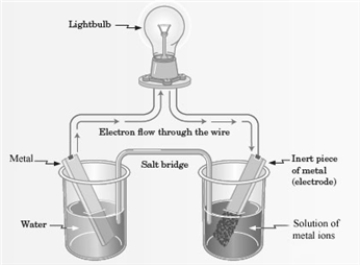 This voltaic cell is represented by the following reaction: Mg(s) + Cu2+(aq) → Cu(s) + Mg2+(aq)
This voltaic cell is represented by the following reaction: Mg(s) + Cu2+(aq) → Cu(s) + Mg2+(aq)
Which of the following is true of the Mg(s) shown in the reaction representing the voltaic cell?
A) It is the anode of the cell.
B) It is the place where reduction occurs.
C) It functions as the oxidizing agent.
D) All of these are correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q81: Which of the following is oxidation?<br>A) loss
Q82: How much does a sample of 5.75
Q83: Which of the following is analogous to
Q84: What is the molar mass of glucose,
Q85: A certain protein has a molar mass
Q87: How many moles of marble, CaCO<sub>3</sub>, are
Q88: The metabolism of one mole of glucose,
Q89: A substance weighing 1 kg is initially
Q90: The products of the reaction of phosphoric
Q91: The combustion of one mole of propane,