Multiple Choice
Consider the following Lewis structure. 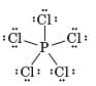 Which of the following correctly characterizes this compound?
Which of the following correctly characterizes this compound?
A) All atoms obey the octet rule.
B) Forty valence electrons are represented.
C) The central atom is from period 4.
D) It contains a metalloid and a nonmetal.
E) None of these are true of this compound.
Correct Answer:

Verified
Correct Answer:
Verified
Q96: How many electrons are associated with a
Q97: In which of the following cases is
Q98: The following represents carbonic acid, an important
Q99: When we name a binary covalent compound,
Q100: The "ide" ending is usually, but not
Q102: If the name of an ion ends
Q103: What is the relationship between the nitrate
Q104: What is the correct name of BaH<sub>2</sub>?<br>A)
Q105: How many electrons are associated with a
Q106: Which of the following molecules is polar?<br>A)