Multiple Choice
What do the two representations have in common? 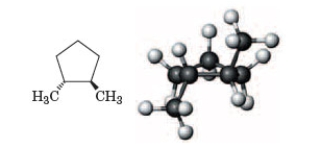
A) Both represent cis isomers of 1,2-dimethylcyclohexane.
B) Both represent cis isomers of 1,2-dimethylcyclopentane.
C) Both represent trans isomers of 1,2-dimethylcyclohexane.
D) Both represent trans isomers of 1,2-dimethylcyclopentane
Correct Answer:

Verified
Correct Answer:
Verified
Q37: What is the common name of 2-methylbutane?<br>A)
Q38: How many different substituent groups can be
Q39: Which of the following is a gas
Q40: Which isomer of hexane has the highest
Q41: How is a tert-butyl group formed?<br>A) by
Q43: Which of the following is soluble in
Q44: Which of the following compounds among 2,3-dimethylpentane,
Q45: Which of the following compounds contains a
Q46: Which of the following statements is false?<br>A)
Q47: Which of the following compounds has isohexane