Multiple Choice
Which is the order of increasing acid strength of the following compounds (least first) ?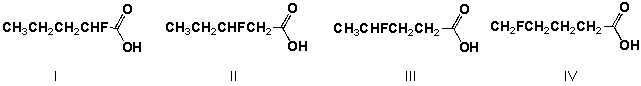
A) I, III, II, IV
B) IV, III, II, I
C) II, I, III, IV
D) IV, III, I, II
Correct Answer:

Verified
Correct Answer:
Verified
Q23: What is the stronger acid in the
Q24: Identify the conjugate acids in the following
Q25: What is the role of diethyl ether
Q26: Water acts as a Brønsted-Lowry base in
Q27: Identify the conjugate bases in the following
Q29: Complete the following reaction.<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8498/.jpg" alt="Complete
Q30: Complete the following reaction.<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8498/.jpg" alt="Complete
Q31: Strong acids have weak conjugate bases.
Q32: Brønsted-Lowry acids accept protons when reacting.
Q33: The stronger acid has the larger (more