Related Questions
Q36: The equilibrium constant will be greater than
Q37: The weakest acid in the following list
Q38: Complete the following reaction.<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8498/.jpg" alt="Complete
Q39: Identify the acid, base, conjugate acid, conjugate
Q40: Identify the Arrhenius acids:<br>I. HCl <br>II. NaOH
Q42: What is the strongest acid in the
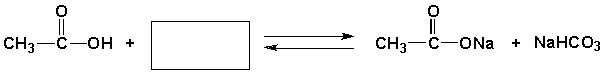
Q43: Complete the following reaction scheme with the
Q44: The higher concentration (reactants or products) at
Q45: Which ion is the strongest base?<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8498/.jpg"
Q46: Arrange the following species in the order