Multiple Choice
What are the correct orbital hybridizations for carbon in the following species?
HINT: Add a lone pair if the charge of the molecule suggests it!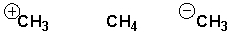
A) CH3+ is sp-hybridized
B) CH3- is sp2-hybridized
C) CH4 is sp2-hybridized
D) CH3- is sp3-hybridized
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: Select the stereoselective reagents from the following
Q14: What is the correct structure for the
Q15: Which reagents react with 2-methyl-1-hexene in a
Q16: Draw the product of this reaction. <img
Q17: Which atom is described by the Lewis
Q19: _ is the number of valence electrons
Q20: Ethynylcyclohexane reacts with sodium amide. The product
Q21: Complete the following reaction by providing the
Q22: The most polar bond in the following
Q23: What is the formal charge of indicated