Multiple Choice
Using Markovnikov's rule, predict the position of the Cl atom in the major product from the reaction of 1-methylcyclohexene with HCl. 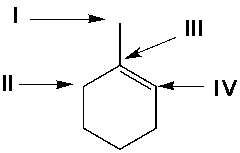
A) I
B) II
C) III
D) IV
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q98: The carbon has the correct orbital hybridization
Q99: Which is the major product from the
Q100: Which of the three molecules testosterone, methadone
Q101: Draw the intermediate of the following reaction.
Q102: Which Lewis structures are correct?<br>HINT: Perform a
Q104: Which statement about contributing structures is false?<br>A)
Q105: Which of the three molecules aspirin, paracetamol
Q106: Using the VSEPR model, predict which molecules
Q107: The following carbocations are listed in increasing
Q108: The hydroboration and subsequent oxidation of 1-heptene