Multiple Choice
Consider the following octahedral complex structures, each involving ethylene diamine and two different, unidentate ligands X and Y. 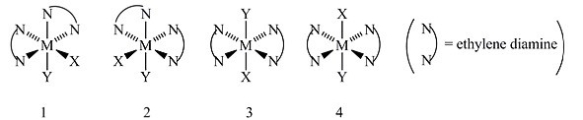 Which, if any, of the following pairs are optical isomers?
Which, if any, of the following pairs are optical isomers?
A) Structures 1 and 2
B) Structures 1 and 3
C) Structures 1 and 4
D) Structures 3 and 4
E) Structures 1, 2, 3, and 4
Correct Answer:

Verified
Correct Answer:
Verified
Q31: The systematic name of the coordination compound
Q38: Which of these square planar complex ions
Q56: In complexes of transition metals, the maximum
Q59: Which of the following ions could exist
Q59: Coordination compounds may exhibit _ and _
Q60: One of the uses of EDTA is
Q65: The following energy-level diagram could correspond to
Q66: How many 4d electrons does a ground-state
Q67: The net rotation of plane-polarized light by
Q68: The atom in a ligand that is