Multiple Choice
Consider the following octahedral complex structures, each involving ethylene diamine and two different, unidentate ligands X and Y. 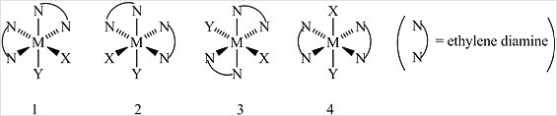 Which one of the following statements about these structures is incorrect?
Which one of the following statements about these structures is incorrect?
A) Structures 1 and 2 are optical isomers.
B) Structures 1 and 3 are optical isomers.
C) Structures 1 and 3 are different complexes.
D) Structures 1 and 4 are geometrical isomers.
E) Structures 3 and 4 are the same complex.
Correct Answer:

Verified
Correct Answer:
Verified
Q24: The maximum oxidation state of an element
Q40: What geometry is particularly common for complexes
Q81: The ion [Co(NH<sub>3</sub>)<sub>6</sub>]<sup>2+</sup> is octahedral and high
Q81: Which of the following ions is most
Q116: An equimolar mixture (50:50 ratio) of two
Q118: How many 3d electrons does a ground-state
Q120: In the coordination compound [Co(en)<sub>2</sub>Cl<sub>2</sub>]Cl, the coordination
Q122: Which is the appropriate energy-level diagram for
Q123: If the plane of polarization is rotated
Q125: What is the systematic name for [CoCl<sub>3</sub>(H<sub>2</sub>O)]<sup>-</sup>?<br>A)