Multiple Choice
Consider the following structures (1 and 2 are octahedral; 3 and 4 are square planar) . 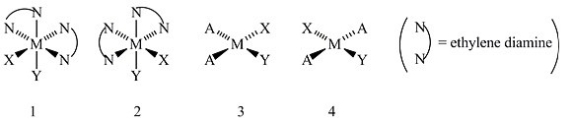 Which one of the following statements about the above structures is correct?
Which one of the following statements about the above structures is correct?
A) Structures 1 and 2 are superimposable.
B) Structures 1 and 2 are geometric isomers.
C) Structures 3 and 4 are structural isomers.
D) Structures 3 and 4 are optical isomers.
E) Structures 3 and 4 are geometric isomers.
Correct Answer:

Verified
Correct Answer:
Verified
Q7: What is the oxidation number of Fe
Q75: How many unpaired electrons are there in
Q76: A bidentate ligand always<br>A) forms bonds to
Q77: What name is given to the species
Q78: In the complex ion [Co(en)<sub>2</sub>Br<sub>2</sub>]<sup>+</sup>, what is
Q81: In the complex ion [Fe(CN)<sub>6</sub>]<sup>4-</sup>, what is
Q82: Cisplatin complexes are<br>A) used in the extraction
Q83: Which of the following ligands is most
Q84: Which of the following metals is capable
Q85: What is the electron configuration of a