Multiple Choice
Which of these electron energy level patterns would absorb light with the shortest wavelength?
A) 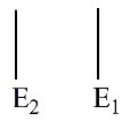
B) 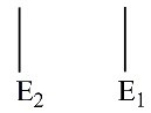
C) 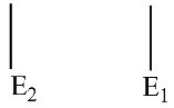
D) 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: The numbers of geometrical isomers and optical
Q30: The oxidation number of Co in [Co(NH<sub>3</sub>)<sub>4</sub>Cl<sub>2</sub>]Cl
Q37: In the coordination compound [Cr(NH<sub>3</sub>)(en)<sub>2</sub>Cl]Br<sub>2</sub>, the coordination
Q41: Which name could correspond to the following
Q42: How many donor atoms enable EDTA to
Q44: What type of structure is exhibited by
Q49: Which are the most common transition metals?<br>A)
Q50: What is the name that refers to
Q51: A complex ion that undergoes a very
Q64: Which of the following ions is least