Multiple Choice
What is E°cell for the following reaction? Al(s) + 3Ag+(aq) → Al3+(aq) + 3Ag(s) 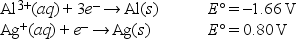
A) -2.46 V
B) 0.86 V
C) -0.86 V
D) 2.46 V
E) 4.06 V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q36: A voltaic cell consists of an Au/Au<sup>3+</sup>
Q38: Which of the following is correct?<br>A) total
Q39: When the following redox equation is balanced
Q40: Consider the following redox equation. Mn(OH)<sub>2</sub>(s) +
Q43: Which electrochemical cell pictured below corresponds to
Q44: Predict the products obtained from the electrolysis
Q46: When the following redox equation is balanced
Q58: What product forms at the cathode during
Q93: In a fuel cell, an external source
Q115: An electroplating solution is made up of