Multiple Choice
What is E°cell for the following reaction? 2 Ag(s) + Sn2+(aq) → 2 Ag+(aq) + Sn(s) 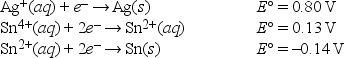
A) +0.94 V
B) -0.94 V
C) +0.67 V
D) -0.67 V
E) +1.34 V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q67: Based on the data presented below, which
Q68: A voltaic cell prepared using aluminum and
Q69: A galvanic cell is constructed using the
Q70: What is the oxidizing agent in the
Q71: Aluminum does not corrode in the same
Q73: What mass of copper can be deposited
Q74: _ is a process used to coat
Q75: The equilibrium constant for the reaction of
Q76: Which is the correct cell notation for
Q77: Complete and balance the following redox equation.