Multiple Choice
What is E°cell for a galvanic cell represented by the combination of the following half-reactions? 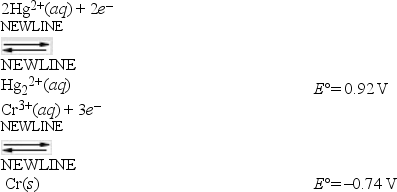
A) -0.18 V
B) 0.18 V
C) 1.28 V
D) 1.66 V
E) 2.12 V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: What is the standard free-energy change for
Q2: If a substance is reduced, it must
Q5: What would you observe if you set
Q7: Consider the reaction in the lead-acid cell
Q8: The redox reaction of peroxydisulfate with iodide
Q9: A salt bridge allows movement of cations
Q10: What is the minimum voltage required for
Q11: The Faraday constant represents the charge of
Q28: Which element is associated with the term
Q54: In the electrolyte of an electrochemical cell,