Multiple Choice
What is E°cell for a galvanic cell represented by the combination of the following half-reactions? 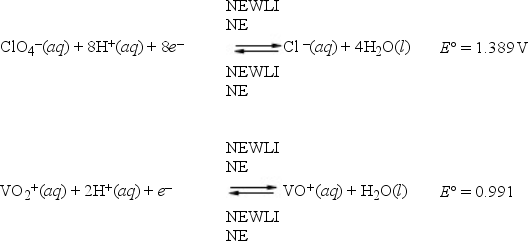
A) -0.398 V
B) -2.380 V
C) 0.398 V
D) 2.380 V
E) 6.539 V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q109: If ΔG° of the following reaction is
Q110: What is ΔG° for the reaction of
Q111: How many minutes would be required to
Q112: The voltaic cell composed of Co(s), Co<sup>2+</sup>(aq),
Q113: Electrons flow to the cathode in a
Q115: What quantity of charge is required to
Q116: Which is the correct cell diagram for
Q117: What is ΔG° at 200°C for the
Q118: What mass of oxygen gas is produced
Q119: _ is the process that uses electrical