Multiple Choice
Based on the data presented below, which is the strongest reducing agent? 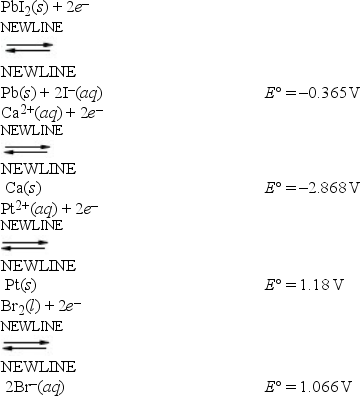
A) Pb(s)
B) Ca(s)
C) Pt(s)
D) Br-(aq)
E) Pt2+(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q101: Which metals may be oxidized by H<sup>+</sup>
Q102: Which is the half-reaction at the cathode
Q103: What is E°<sub>cell</sub> for the following reaction?
Q104: A voltaic cell consists of a Cd/Cd<sup>2+</sup>
Q105: Which statement is correct?<br>A) The cathode is
Q107: Consider the following balanced redox reaction. Mn<sup>2+</sup>(aq)
Q108: Which equation is correct?<br>A) E<sub>cell</sub> = RT
Q109: If ΔG° of the following reaction is
Q110: What is ΔG° for the reaction of
Q111: How many minutes would be required to