Multiple Choice
What is ΔG° for the following reaction? (F = 96,500 C • mol -1) I2(s) + 2Br-(aq) → 2I-(aq) + Br2(l)
Given: 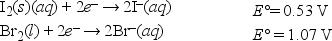
A) +104 kJ/mol
B) -104 kJ/mol
C) +309 kJ/mol
D) +52 kJ/mol
E) -52 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: The Faraday constant represents the charge of
Q13: A cell can be prepared from copper
Q14: Which is not a redox reaction?<br>A) Al(OH)<sub>4</sub><sup>-</sup>(aq)
Q15: Complete and balance the following redox equation
Q17: A metal object is to be gold-plated
Q18: How much charge must pass through an
Q19: At equilibrium E° = 0.
Q20: What is the equilibrium constant at 25°C
Q21: Which component of the following cell is
Q57: What product forms at the anode during