Multiple Choice
What is ΔS° for the following reaction? SiCl4(g) + 2Mg(s) → 2MgCl2(s) + Si(s) 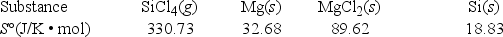
A) -254.96 J/K • mol
B) -198.02 J/K • mol
C) +198.02 J/K • mol
D) +254.96 J/K • mol
E) +471.86 J/K • mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: a. Explain what is meant by a
Q82: Which species will have the greatest absolute
Q91: What is ΔG°<sub>rxn</sub> for the combustion of
Q92: According to which scientific law does the
Q93: The element oxygen was prepared by Joseph
Q95: Hydrogen sulfide decomposes according to the following
Q98: Calculate ΔG° for the combustion of ethanol
Q99: Which, if any, of the following processes
Q100: Which is true for a system at
Q101: Which is necessary for a process to