Multiple Choice
A 20.0-mL sample of 0.30 M HClO was titrated with 0.30 M NaOH. The following data were collected during the titration. Determine to Ka for HClO. 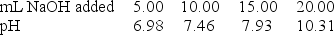
A) 1.1 × 10-7
B) 3.5 × 10-8
C) 1.2 × 10-8
D) 4.9 × 10-11
E) None of the answers is correct.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: Which pair of substances could form a
Q14: What is the pH of a buffer
Q15: A wildlife biologist is interested in testing
Q16: A(n) _ is an ion containing a
Q19: When a strong acid is titrated with
Q20: An acetic acid buffer containing 0.50 M
Q21: Which will precipitate first when AgNO<sub>3</sub> is
Q22: What molar ratio of CH<sub>3</sub>COOH to CH<sub>3</sub>COONa
Q23: The solubility of magnesium phosphate is 2.27
Q24: Bromothymol blue is a common acid-base indicator.It