Multiple Choice
Which one of the following is the best representation of the titration curve which will be obtained in the titration of a weak diprotic acid H2A (0.10 M) with a strong base of the same concentration?
A) 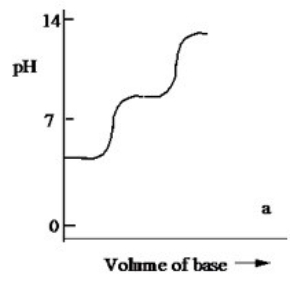
B) 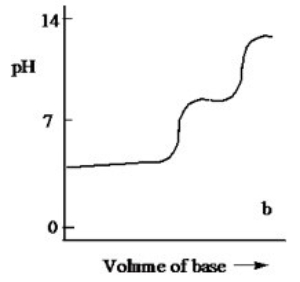
C) 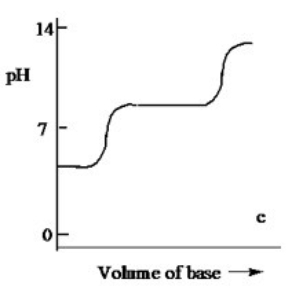
D) 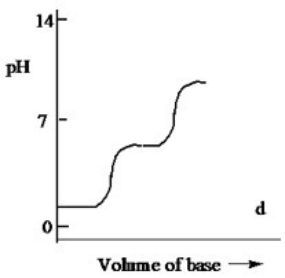
E) 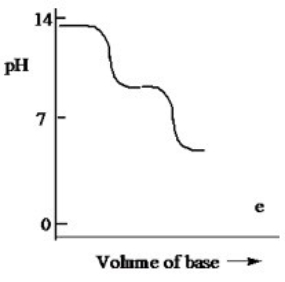
Correct Answer:

Verified
Correct Answer:
Verified
Q30: A CH<sub>3</sub>COOH/CH<sub>3</sub>COO<sup>-</sup> buffer can be produced by
Q60: Increasing the concentrations of the components of
Q70: For the 1:1 ratio combination of a
Q71: What is the Henderson-Hasselbach equation?<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8482/.jpg"
Q72: A 50.00-mL solution of 0.10 M HNO<sub>2</sub>
Q74: What is the [H<sub>3</sub>O<sup>+</sup>] in a solution
Q76: You have 500.0 mL of a buffer
Q77: The indicator propyl red has K<sub>a</sub> =
Q78: Suppose 0.015 mol of KOH is added
Q79: Calculate the minimum concentration of Mg<sup>2+</sup> that