Multiple Choice
Below is a representation of an aqueous solution of a weak acid HA at equilibrium. (Each circle represents 1.0 ×10-3 mol of atoms, and the volume of the box is 1.0 L. Solvent water molecules are not shown for clarity.) 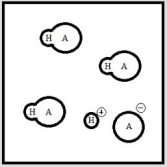 What is the pH of the solution?
What is the pH of the solution?
A) 2.4
B) 3.0
C) 2.1
D) 11.0
E) 11.6
Correct Answer:

Verified
Correct Answer:
Verified
Q45: All strong acids have weak conjugate bases.
Q67: Which is the formula for the hydronium
Q73: HCN is classified as a weak acid
Q80: Which is the most basic oxide?<br>A) Bi<sub>2</sub>O<sub>3</sub><br>B)
Q81: Which aqueous solution has the highest pH?<br>A)
Q82: What is the concentration of OH<sup>-</sup> in
Q84: Farmers who raise cotton once used arsenic
Q86: Which of the following species can act
Q87: The following is the correct order for
Q89: What is the name given to a