Multiple Choice
Below is a representation of an aqueous solution of a weak acid HA at equilibrium. (Each circle represents 1.0 ×10-3 mol of atoms, and the volume of the box is 1.0 L. Solvent water molecules are not shown for clarity.) 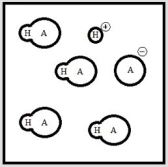 What is Ka of HA?
What is Ka of HA?
A) 1.0 ×10-6
B) 6.0 ×10-3
C) 2.5 ×10-4
D) 3.6 ×10-5
E) 1.0 ×10-9
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q60: What is the value of the equilibrium
Q61: The pH of a Ba(OH)<sub>2</sub> solution is
Q62: After a substance acting as a strong
Q63: What is [OH<sup>-</sup>] for a solution at
Q64: What mass of sodium nitrite must be
Q66: Calculate the pH of a 0.021 M
Q67: Find the pH of a 0.135 M
Q68: Which is a Lewis acid but not
Q69: Consider the weak bases below and their
Q120: Which one of these salts will form