Multiple Choice
Which represents a weak base B with Kb= 2 ×10-3? (Each circle represents 1.0 ×10-3 mol of atoms, and the volume of each box is 1.0 L. Solvent water molecules are not shown for clarity.)
A) 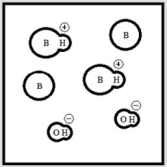
B) 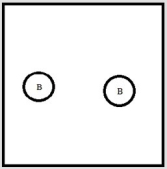
C) 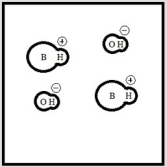
D) 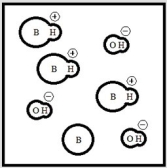
E) 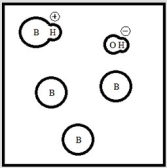
Correct Answer:

Verified
Correct Answer:
Verified
Q12: What is the conjugate acid of CO<sub>3</sub><sup>2-</sup>
Q13: What is the conjugate base of water?<br>A)
Q14: A 0.15 M solution of chloroacetic acid
Q15: Which is the correct statement?<br>A) A polyprotic
Q16: A solution is prepared by adding 0.10
Q18: Find the pH of a 0.20 M
Q19: Which is a basic oxide?<br>A) CO<sub>2</sub><br>B) MgO<br>C)
Q20: For H<sub>3</sub>PO<sub>4</sub>, K<sub>a1</sub> = 7.3 × 10<sup>-3</sup>,
Q21: In the reaction, HSO<sub>4</sub><sup>-</sup>(aq) + OH<sup>-</sup>(aq) <img
Q22: What are the products of hydrolysis of