Multiple Choice
Below is a representation of an aqueous solution of a weak acid HA at equilibrium. (Each circle represents 1.0 mmol of atoms, and the volume of the box is 1.0 L. Solvent water molecules are not shown for clarity.) 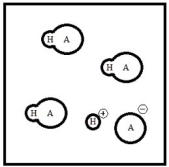 What is the percent ionization of HA?
What is the percent ionization of HA?
A) 100%
B) 50%
C) 25%
D) 12.5%
E) Cannot be calculated without knowledge of Ka
Correct Answer:

Verified
Correct Answer:
Verified
Q78: A solution is prepared by adding 0.10
Q123: Carboxylic acids are _.<br>A) always weak acids<br>B)
Q124: Identify the conjugate base of HPO<sub>4</sub><sup>2-</sup> in
Q125: Butyric acid is responsible for the odor
Q126: Give the two factors that influence the
Q127: What is the pH of a 0.056
Q129: Which is a Lewis acid?<br>A) CH<sub>3</sub>NH<sub>2</sub><br>B) BCl<sub>3</sub><br>C)
Q130: For H<sub>3</sub>PO<sub>4</sub>, K<sub>a1</sub> = 7.3 × 10<sup>-3</sup>,
Q131: Which is the strongest acid?<br>A) SO<sub>4</sub><sup>2-</sup><br>B) H<sub>2</sub>SO<sub>3</sub><br>C)
Q132: The substance HClO<sub>4</sub> is considered to be<br>A)