Multiple Choice
A sample of solid naphthalene is introduced into an evacuated flask. Using the data below, what is the equilibrium vapor pressure of naphthalene (C10H8) in the flask at 35°C? 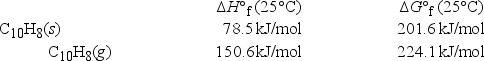
A) 890. mmHg
B) 0.22 mmHg
C) 696 mmHg
D) 0.086 mmHg
E) 833 mmHg
Correct Answer:

Verified
Correct Answer:
Verified
Q40: Which equation is correct?<br>A) ΔG = ΔG°
Q42: At 25°C, the equilibrium constant, K<sub>c</sub>, for
Q43: At 450°C, tert-butyl alcohol decomposes into water
Q44: What is used to explain the effect
Q46: The solubility product constant, K<sub>sp</sub>, at 25°C
Q47: What is defined as a fraction with
Q48: At 340. K, K<sub>P</sub> = 69 for
Q49: When the reaction 2H<sub>2</sub>S(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8482/.jpg" alt="When
Q50: At 850°C, the equilibrium constant, K<sub>P</sub>, for
Q132: At equilibrium, the rate of the forward