Multiple Choice
For the endothermic reaction A2(g)  2A(g) , a snapshot of an equilibrium mixture of A(g) and A2(g) at low temperature may look as follows. (Each circle represents 1.0 mol of A atoms, and the volume of the box is 1.0 L.)
2A(g) , a snapshot of an equilibrium mixture of A(g) and A2(g) at low temperature may look as follows. (Each circle represents 1.0 mol of A atoms, and the volume of the box is 1.0 L.) 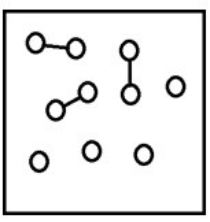 If the system pressure is lowered, what might the new equilibrium system look like?
If the system pressure is lowered, what might the new equilibrium system look like?
A) 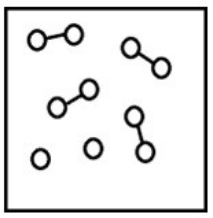
B) 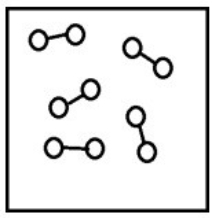
C) 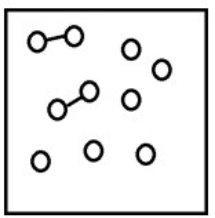
D) 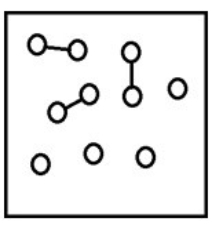
E) 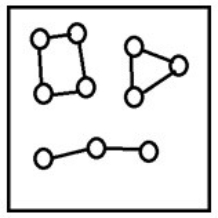
Correct Answer:

Verified
Correct Answer:
Verified
Q75: Which is the correct mass-action expression, Q<sub>c</sub>,
Q76: The equilibrium constant, K<sub>c</sub>, for the reaction
Q77: Suppose 4.2 mol of oxygen and 4.0
Q78: Which equation is correct?<br>A) ΔG = ΔG°
Q79: The equilibrium constant, K<sub>P</sub>, for the reaction
Q81: The observation that at equilibrium, the reaction
Q82: Hydrogen peroxide (H<sub>2</sub>O<sub>2</sub>) decomposes according to the
Q83: Increasing the temperature of an exothermic reaction
Q84: The equilibrium constant expression for the reaction<br>CuO(s)
Q85: Consider the equilibrium equation C(s) + O<sub>2</sub>(g)