Multiple Choice
Consider the following potential energy profile for the A → B reaction. How many elementary steps are there? 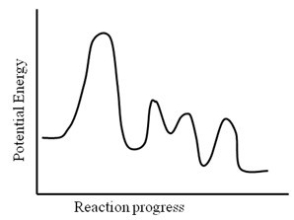
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: For a zeroth-order reaction, if the concentration
Q10: Carbon-14 is a radioactive isotope which decays
Q11: The first-order reaction SO<sub>2</sub>Cl<sub>2</sub>→ SO<sub>2</sub> + Cl<sub>2</sub>
Q12: For the reaction 3A(g) + 2B(g) →
Q13: The reaction A + 2B → Products
Q15: Which of the following is the correct
Q16: The data below were determined for the
Q17: The radioactive isotope tritium decays with a
Q18: The oxidation of iodide ions by arsenic
Q19: The rate law for the rearrangement of