Multiple Choice
The following diagram represents the zeroth-order decomposition of A to form X according to the following balanced chemical equation: A → X. Each sphere represents 1.0 mmol of atoms, and the volume of the box is 1.0 L. 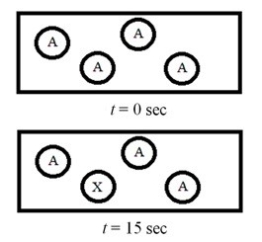 What is the half-life of the reaction?
What is the half-life of the reaction?
A) 15 s
B) 30 s
C) 36 s
D) 45 s
E) 60 s
Correct Answer:

Verified
Correct Answer:
Verified
Q18: Is a bimolecular reaction necessarily second-order? Is
Q73: According to the _ _ of chemical
Q74: The reaction A + 2B → Products
Q75: The rate law for the reaction 3A
Q76: Butadiene, C<sub>4</sub>H<sub>6</sub> (used to make synthetic rubber
Q77: Sucrose decomposes to fructose and glucose in
Q79: What statement below best describes the graph
Q80: The _ is the rate for a
Q81: At 25°C the rate constant for the
Q83: What is the name given for the