Multiple Choice
The following diagram represents the first-order decomposition of A to form X according to the following balanced chemical equation: A → X. Each sphere represents 1.0 mmol of atoms, and the volume of the box is 1.0 L. 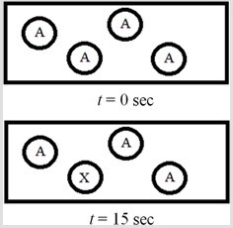 What is the half-life of the reaction?
What is the half-life of the reaction?
A) 15 s
B) 30 s
C) 36 s
D) 45 s
E) 60 s
Correct Answer:

Verified
Correct Answer:
Verified
Q9: For a zeroth-order reaction, if the concentration
Q32: The rate of a reaction is determined
Q89: A(n) _ increases the reaction rate without
Q90: Consider the reaction 2NH<sub>3</sub>(g) → N<sub>2</sub>(g) +
Q92: A reaction has the following rate law:
Q93: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8482/.jpg" alt=" A)
Q96: What rate constant can be determined from
Q97: What is the integrated rate law for
Q98: Consider the following potential energy profile for
Q99: What is the molecularity of the following