Multiple Choice
Which is true concerning the following potential energy diagram? 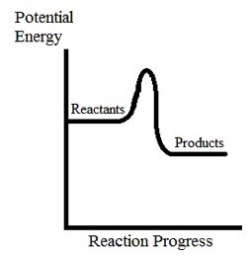
A) This reaction is exothermic and has a positive activation energy.
B) This reaction is endothermic and has a positive activation energy.
C) This reaction is exothermic and has a negative activation energy.
D) This reaction is endothermic and has a negative activation energy.
E) This reaction is thermoneutral and has a zero activation energy.
Correct Answer:

Verified
Correct Answer:
Verified
Q32: The rate of a reaction is determined
Q56: The units of the rate constant depend
Q96: What rate constant can be determined from
Q97: What is the integrated rate law for
Q98: Consider the following potential energy profile for
Q99: What is the molecularity of the following
Q101: For the chemical reaction A → C,
Q102: The rate law for the reaction 3A
Q103: The oxidation of iodide ions by arsenic
Q104: The _ is the name given to