Multiple Choice
The following is an Arrhenius plot of a first-order reaction. The rate constant is measured in units of s-1. 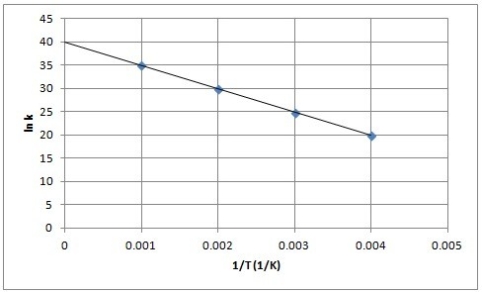 Based on this Arrhenius plot, what is the Arrhenius frequency factor (A) of the reaction? (R = 8.314 J/K mol)
Based on this Arrhenius plot, what is the Arrhenius frequency factor (A) of the reaction? (R = 8.314 J/K mol)
A) 5.0 s-1
B) 40 s-1
C) 50 s-1
D) 5.0 × 103 s-1
E) 2.4 × 1017 s-1
Correct Answer:

Verified
Correct Answer:
Verified
Q9: The units of the rate of reaction
Q9: Ammonium cyanate (NH<sub>4</sub>CNO) reacts to form urea
Q61: For the hypothetical reaction A + 3B
Q62: For the reaction X + Y →
Q63: What is the half-life for a second-order
Q65: The activation energy for the following first-order
Q68: Consider the reaction 2NOBr(g) → 2NO(g) +
Q69: The reaction 2NO<sub>2</sub>(g) → 2NO(g) + O<sub>2</sub>(g)
Q70: A certain first-order reaction A → B
Q71: What is the integrated rate law for