Multiple Choice
Below is a diagram representing a solvent A(l) in a 1-L beaker, and a solute X dissolved in the solvent. Solvent A has a density of 0.8 g/mL, and a molar mass of 40 g/mol. Solute X has a molar mass of 30 g/mol. Each circle of X represents 1 mol of X. Assume that the solute addition does not significantly change the volume of liquid in the beaker. 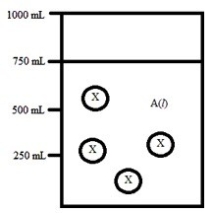 What is the mole fraction of the solute X in this solution?
What is the mole fraction of the solute X in this solution?
A) 0.67
B) 0.53
C) 0.27
D) 0.21
E) 0.04
Correct Answer:

Verified
Correct Answer:
Verified
Q88: Explain the following, on the basis of
Q141: An electrolyte is a substance that dissolves
Q142: What is the osmotic pressure of a
Q143: _ is the value for i, the
Q145: 1.00 L of an aqueous solution contains
Q146: What is the name given to a
Q147: What name is given to the major
Q148: Below is a diagram representing a solvent
Q150: The mole fraction of potassium nitrate in
Q151: What term describes the process when two