Multiple Choice
Diethyl ether, (CH3CH2) 2O, used as a solvent for extraction of organic compounds from aqueous solutions, has a high vapor pressure, which makes it a potential fire hazard in laboratories in which it is used. How much energy is released when 100.0 g is cooled from 53.0°C to 10.0°C? 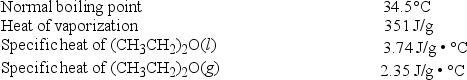
A) 10.1 kJ
B) 16.1 kJ
C) 21.6 kJ
D) 35.1 kJ
E) 48.6 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q50: Identify the dominant (strongest)type of intermolecular force
Q85: Ethanol (C<sub>2</sub>H<sub>5</sub> - OH) will have a
Q93: The maximum number of phases of a
Q124: The molar enthalpy of vaporization of hexane
Q125: Which of the following statements is true?<br>A)
Q127: What quantity of heat is required to
Q130: _ _ are solids that lack a
Q131: Which statement concerning the vapor pressures of
Q133: _ is the name given to the
Q134: Which would be expected to have the