Multiple Choice
Below is the phase diagram for sulfur. Which phase has the highest density at 119°C? 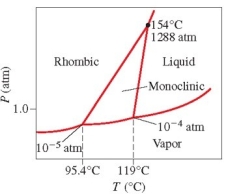
A) liquid
B) vapor
C) rhombic solid
D) monoclinic solid
E) liquid-vapor boundary line
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q42: The arrow in this phase diagram represents
Q43: Which is not a valid phase diagram
Q44: Which substance should exhibit hydrogen bonding in
Q45: _ is the attraction of unlike molecules
Q46: Palladium crystallizes in a face-centered cubic unit
Q48: Ionic crystals are composed of charged spheres
Q49: Naphthalene sublimes at room temperature and 1
Q50: The strongest intermolecular interactions between hydrogen sulfide
Q51: If the adhesive forces between a liquid
Q52: A metal such as chromium in the