Multiple Choice
Based on the phase diagram of a pure substance given below, what change of state occurs as the substance changes from point C to point D? 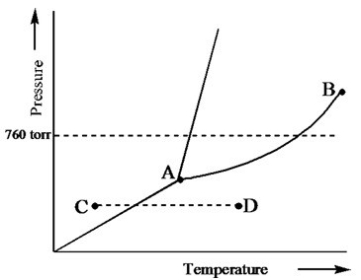
A) The substance increases in temperature with a phase change from solid to liquid.
B) The substance increases in temperature with a phase change from solid to vapor.
C) The substance increases in temperature with a phase change from liquid to vapor.
D) The substance increases in temperature but does not undergo a phase change.
E) The substance increases in temperature and becomes a supercritical fluid.
Correct Answer:

Verified
Correct Answer:
Verified
Q8: _ _ are characterized by an instantaneous
Q9: What name is given to the curved
Q11: Based on the phase diagram of a
Q12: Based on the phase diagram of a
Q14: Which is the correct equation for the
Q15: At a temperature of 27 K, neon
Q17: In a sample of hydrogen iodide, _
Q27: Which one of the following crystallizes in
Q42: Identify the dominant (strongest)type of intermolecular force
Q91: The shape of the water-to-glass meniscus results