Multiple Choice
Based on the phase diagram of a pure substance given below, what phase exists at point F? 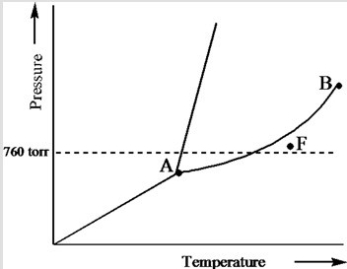
A) vapor + liquid
B) vapor
C) liquid
D) solid
E) supercritical fluid
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q21: Helium atoms do not combine to form
Q113: Which one of the following substances does
Q114: At what temperature does ethanol boil on
Q115: The vapor pressure of ethanol is 400.
Q116: Which of the following atoms does not
Q117: Poor conductor of heat and electricity, soft,
Q119: Which of the following factors can contribute
Q120: Below is the phase diagram for sulfur.
Q122: _ _ is another name given for
Q123: For the solid forms of the following