Essay
Consider the phase diagram shown below. 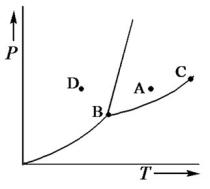 a. What phase(s) is/are present at point A?
a. What phase(s) is/are present at point A?
b. What phase(s) is/are present at point B?
c. Name point C and explain its significance.
d. Starting at D, if the pressure is lowered while the temperature remains constant, describe what will happen.
Correct Answer:

Verified
a. liquid
b. solid, liquid, and gas
c. C...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
b. solid, liquid, and gas
c. C...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q19: The energy required to increase the surface
Q20: How many phase changes are represented in
Q22: Liquid ammonia can be used as a
Q23: Solids are generally most stable in crystalline
Q25: Which one of the following pure substances
Q26: Which substance has the lowest vapor pressure
Q27: The strongest intermolecular interactions between pentane (C<sub>5</sub>H<sub>12</sub>)
Q27: What name is given to the phenomenon
Q28: Which of the following pure substances would
Q60: The strongest intermolecular interactions between ethyl alcohol