Multiple Choice
What is the molecular geometry of XeO2F2 as predicted by the VSEPR model? 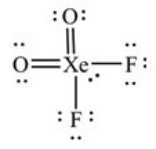
A) square planar
B) tetrahedral
C) square pyramidal
D) seesaw
E) octahedral
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: The angles between sp<sup>2</sup> hybrid orbitals are
Q8: How do you describe the molecular geometry
Q9: What is the molecular geometry of N<sub>2</sub>O
Q10: Carbon uses _ hybrid orbitals in ClCN.<br>A)
Q11: In the valence bond treatment, a π
Q12: The nitrosonium ion, NO<sup>+</sup>, forms a number
Q14: Which is true for carbon monoxide?<br>A) CO
Q15: To make an sp<sup>3</sup> hybrid orbital, one
Q17: What is the molecular geometry of NO<sub>2</sub><sup>-</sup>
Q18: The Lewis structure of formaldehyde, CH<sub>2</sub>O, is