Multiple Choice
For a homonuclear diatomic molecule, which molecular orbital is the lowest in energy?
A) 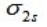
B) 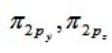
C) 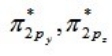
D) 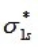
E) All of these orbitals have the same energy.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: A molecule which contains polar bonds will
Q42: Consider the species F<sub>2</sub><sup>+</sup>, F<sub>2</sub>, and F<sub>2</sub><sup>-</sup>.
Q43: Valence bond theory predicts that carbon will
Q44: What does the abbreviation VSEPR stand for?<br>A)
Q46: Which is the most reasonable prediction for
Q48: According to the VSEPR model, what is
Q49: What is the number of lone electron
Q50: Which statement about orbital hybridization is incorrect?<br>A)
Q51: Use the VSEPR model to predict the
Q52: According to the VSEPR model, what is