Multiple Choice
Which figure best illustrates the hybrid orbitals on carbon in benzene, C6H6?
A) 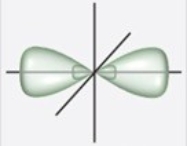
B) 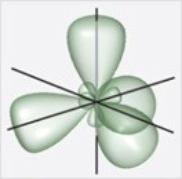
C) 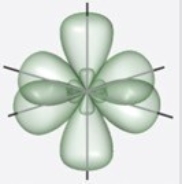
D) 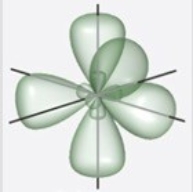
E) 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q34: What is the number of lone electron
Q36: Consider the species N<sub>2</sub><sup>-</sup>, N<sub>2</sub>, and N<sub>2</sub><sup>+</sup>.
Q37: What is the molecular geometry of SiF<sub>6</sub><sup>2-</sup>
Q38: Atoms of period 3 and beyond can
Q38: The BrF<sub>5</sub> molecule has polar bonds and
Q41: What is the molecular geometry of the
Q42: Consider the species F<sub>2</sub><sup>+</sup>, F<sub>2</sub>, and F<sub>2</sub><sup>-</sup>.
Q43: Valence bond theory predicts that carbon will
Q44: What does the abbreviation VSEPR stand for?<br>A)
Q53: In one sentence state the basic principle