Multiple Choice
Which figure best illustrates the hybrid orbitals on phosphorous in PH3?
A) 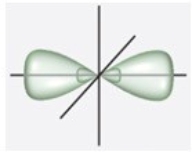
B) 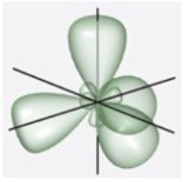
C) 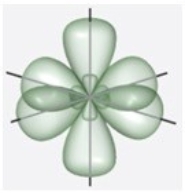
D) 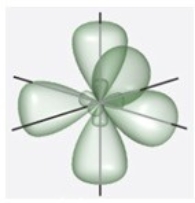
E) 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: The Lewis structure of formaldehyde, CH<sub>2</sub>O, is
Q19: Which is the most reasonable prediction for
Q20: According to the VSEPR model, a molecule
Q21: What is the predicted O-C-O bond angle
Q22: According to molecular orbital theory, what is
Q24: What is the number of lone electron
Q25: Indicate the type of hybrid orbitals used
Q26: Which molecular formula corresponds to a structural
Q27: What is the total number of electron
Q28: How many electron domains are on the